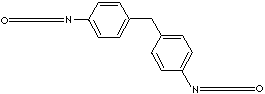
| 4,4'-DIPHENYLMETHANE DIISOCYANATE | |||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
|||||||||||||||||||||||||||||||||||||
| CAS NO. | 101-68-8 |
| |||||||||||||||||||||||||||||||||||
| EINECS NO. | 202-966-0 | ||||||||||||||||||||||||||||||||||||
| FORMULA | CH2(C6H4NCO)2 | ||||||||||||||||||||||||||||||||||||
| MOL WT. | 250.25 | ||||||||||||||||||||||||||||||||||||
| H.S. CODE | |||||||||||||||||||||||||||||||||||||
|
TOXICITY |
|||||||||||||||||||||||||||||||||||||
| SYNONYMS | MDI; MDR; Methylbisphenyl isocyanate; | ||||||||||||||||||||||||||||||||||||
| 4,4'-Diphenylmethane diisocyanate; Methylenebis(4-phenylisocyanate); 1,1'-Methylenebis(4-isocyanatobenzene); 4,4'-Diisocyanatodiphenylmethane; 4,4'-Diphenylmethane diisocyanate; 4,4'-Diphenylmethanediisocyante; 4,4'-Methylenebis(phenyl isocyanate); 4,4'-Methylenedi-p-phenylene diisocyanate; 4,4'-Methylenediphenyl diisocyanate; 4,4'-Methylenediphenyl isocyanate; 4,4'-Methylenediphenylene isocyanate; 4-4'-Diisocyanate de diphenylmethane; Benzene, 1,1'-methylenebis(4-isocyanato-; Bis(1,4-isocyanatophenyl)methane; Bis(4-isocyanatophenyl)methane; Bis(p-isocyanatophenyl)methane; Bis(para-isocyanatophenyl)methane; Di(4-isocyanatophenyl)methane; Difenil-metan-diisocianato; Difenylmethaan-dissocyanaat; Diphenyl methane diisocyanate; Diphenylmethan-4,4'-diisocyanat; Diphenylmethane diisocyanate; Diphenylmethyl diisocyanate; Isocyanic acid, ester with diphenylmethane; Isocyanic acid, methylenedi-p-phenylene ester; Isonate 125 MF; Isonate 125M; Methylene bisphenyl isocyanate; Methylene di-p-phenylene isocyanate; Methylenebis(4-isocyanatobenzene); Methylenebis(4-phenyl isocyanate); Methylenebis(4-phenylene isocyanate); Methylenebis(p-phenyl isocyanate); Methylenebis(p-phenylene isocyanate); Methylenebis(para-phenyl isocyanate); Methylenebis(para-phenylene isocyanate); Methylenedi-p-phenylene diisocyanate; Methylenedi-para-phenylene diisocyanate; p,p'-Diphenylmethane diisocyanate; p,p'-Methylenebis(phenyl isocyanate); para,para'-Diphenylmethane diisocyanate; para,para'-Methylenebis(phenyl isocyanate); 1,1'-Methylenebis(4-isocyanatobenzene); 4,4'-Methylenediphenyl diisocyanate; 4,4'-Methylenediphenylene diisocyanate; Benzene, 1,1'-methylenebis(4-isocyanato- Isocyanic acid, methylenedi-p-phenylene ester; 1,1'-Methylenebis(4-isocyanatobenzene); 4,4'-Diphenylmethane diisocyanate; 4,4'-Methylenediphenyl diisocyanate; Benzene, 1,1'-methylenebis(4-isocyanato-; Diphenylmethane diisocyanate; Diphenylmethane-4,4'-diisocyanate; Isocyanic acid, methylenedi-p-phenylene ester; Methylene bisphenyl isocyanate; Methylene diphenyl diisocyanate; Methylenebis(phenylisocyanate | |||||||||||||||||||||||||||||||||||||
| OTHER CAS RN |
53633-14-0; 55157-41-0; 57460-66-9; 77090-48-3; 88001-94-9; 97568-33-7; 142690-07-1;153986-89-1; 201528-77-0; |
||||||||||||||||||||||||||||||||||||
|
DERIVATION |
|
||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | white to light yellow crystals | ||||||||||||||||||||||||||||||||||||
| MELTING POINT | 40 C | ||||||||||||||||||||||||||||||||||||
| BOILING POINT |
314 C | ||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 1.23 | ||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Reacts | ||||||||||||||||||||||||||||||||||||
| pH |
| ||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | |||||||||||||||||||||||||||||||||||||
|
AUTOIGNITION |
| ||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
| ||||||||||||||||||||||||||||||||||||
|
NFPA RATINGS |
|||||||||||||||||||||||||||||||||||||
| FLASH POINT |
212 | ||||||||||||||||||||||||||||||||||||
| STABILITY | |||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
|||||||||||||||||||||||||||||||||||||
Diisocyanates (or polyisocyanates) are monomers for polyurethane production.
Polyurethane is made from a variety of diisocyanates in conjunction with
polyether and polyester polyols as co-reactants by addition polymerization which
needs at least two -N=C=O groups. Polyurethanes are widely used in the
manufacture of flexible and rigid foams, fibres, coatings, and elastomers. The
most common diisocyantes for this reaction are:
An aromatic diisocyanate Methylene diphenyl diisocyanate ( MDI) is the most consumed diisocyanate used as a major raw material used in the production of rigid polyurethane foam and polyurethane elastomers and strength adhesive. Typically, one tonne of polyurethane foam needs 0.616 tonne of MDI and balance tonne of polyols (such as polyester and polyether), with small amount blowing agent. MDI exists in three isomers, 2,2'-, 2,4'-, and 4,4'-MDI. The 4,4' isomer, a symmetrical molecule having equal reactivity of two groups, is practically useful. End application include foam insulation, refrigeration, coatings, adhesives, sealants and elastomers.. |
|||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | |||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
|||||||||||||||||||||||||||||||||||||
| PURITY |
| ||||||||||||||||||||||||||||||||||||
| N=C=O CONTENT |
| ||||||||||||||||||||||||||||||||||||
|
COLOR, APHA |
| ||||||||||||||||||||||||||||||||||||
|
NCO EQUVALENT WT. |
| ||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | |||||||||||||||||||||||||||||||||||||
| PACKING | |||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | |||||||||||||||||||||||||||||||||||||
| UN NO. | 2489 | ||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | |||||||||||||||||||||||||||||||||||||
| Hazard Symbols: XN, Risk Phrases: 20-36/37/38-42/43, Safety Phrases: (2-)-23-36/37-45 | |||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF CYANATE (ISOCYANATE) |
|||||||||||||||||||||||||||||||||||||
Cyanic acid
(the isomer of fulminic acid) is an unstable (explosive), poisonous, volatile,
clear liquid with the structure of H-O-C��N (the oxoacid formed from the
pseudohalogen cyanide), which is readily converted to
cyamelide and fulminic
acid. There is another isomeric cyanic acid with the
structure of H-N=C=O, called isocyanic acid. Cyanate group (and isocyanate group) can react with itself.
Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with
the structure of [HOC(NCOH)2N], is the trimer
of cyanic acid. The
trimer
of isocyanic acid
is called biuret.
Cyanic acid hydrolyses to ammonia and carbon dioxide in water. The salts and esters of cyanic acid are cyanates. But esters of normal cyanic acid are not known. The salts and esters of isocyanic acid are isocyanates. The isocyanate group reacts with the hydroxyl functional group to form a urethane linkage. Diisocyanates (or polyisocyanates) are monomers for polyurethane production. Polyurethane is made from a variety of diisocyanates in conjunction with polyether and polyester polyols as co-reactants by addition polymerization which needs at least two -N=C=O groups. Polyurethanes are widely used in the manufacture of flexible and rigid foams, fibres, coatings, and elastomers. If isocyanate monomer is polymerized with amine group, polyurea is produced. Cyanates (or Isocyanates) are readily reacts with various form of amine (including ammonia, primary-, secondary-amines, amides and ureas) and hydroxyl functional group. They are used in the synthesis for the target molecules such as pharmaceuticals, pesticides, textile softener, lubricants and industrial disinfectants. They can convert to polycyclic compounds such as hydantoins and imidazolons. They are used as plastic additives and as heat treatment salt formulations for metals. |
|||||||||||||||||||||||||||||||||||||